by Walt Maclay, President of Voler Systems, PRG Member
Are you on your way to market with a new hardware product? Do you want to know if your product is feasible for development, prototyping, and scale?
Case Study – Annuvia
Background
Annuvia, Inc. provides health, safety, emergency preparedness and response training to organizations across America. The company sells and services automated external defibrillator (AED) units, which are used to save sudden cardiac arrest (SCA) patients. They also offer the Arch AED Medical Direction and Oversight software, proprietary software that saves time and removes compliance obstacles to AED ownership.
Market Need
AED devices are only useful if they are in working order when a SCA incident occurs. Unfortunately, periodic manual inspection by AED owners is unreliable. Micah Bongberg, President and CEO of Annuvia, thought of a new product idea – a device that could constantly monitor the battery life and working status of AED units and notify owners of the device status automatically.
Why do a Feasibility Assessment?
Mr. Bongberg wanted to take his hardware device from concept to prototype quickly and within a limited budget. However, he did not know what it would take to develop, prototype and commercialize this new hardware product, and wanted to identify, understand, and solve risks early.
What disciples were involved?
The feasibility assessment performed was a four week “sprint” to explore design options that would best meet market needs, technical specifications, and costs. The feasibility assessment delivered a structured approach to determine if the initial concept could be developed, and what were the best alternatives available. For this project, we leveraged Voler Systems for electrical and software, Fusion Design for mechanical, and Adolph Consulting Services (ACS) for operations and supply chain feasibility.
How did Feasibility help?
Performing a feasibility study early helped Micah to lower his investment, solve 3 development risks, and mitigate these risks prior to going into full scale development (which would have required several hundred thousand dollars of investment).
What are Typical Feasibility Risks?
- Market: Do customers want your product? Does it include key features? Is it competitively priced?
- Technology: Can it technically meet the need at the right cost?
- Regulatory: Are you compliant with the many government standards and regulations both in the United States and Internationally?
- Supply Chain: Where are you going to buy your components? Have you considered issues such as relying on a sole source, component obsolescence, long lead times, and changing costs?
- Manufacturing / Fulfillment: How much will it cost to assemble and test your design? What is the defect rate? Do you have a fulfillment and repair strategy?
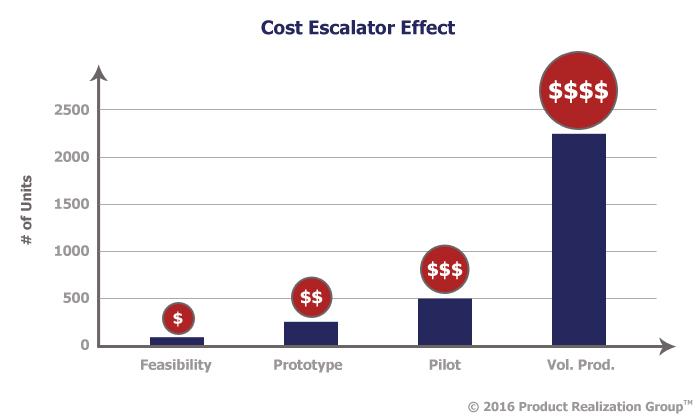
Costs for mistakes escalate substantially when moving from feasibility to prototype to pilot and again from pilot to full production. A feasibility study done at the right time can catch these problems before they escalate.
Solving 3 Hardware Development Risks
Working in a four week sprint, we identified and solved three development risks in the project including:
- Regulatory
- Technology
- Supply Chain
#1: Regulatory
FDA Medical Device Classification
Initially we thought that the new product would be considered a Class II medical device, which would have added $100 K to the development budget and increased the schedule by six months.
With the help of our Regulatory expert, Dr. Geetha Rao, we developed a development strategy whereby the housing would not directly contact the AED, and thus avoided the need for FDA compliance altogether. During her correspondence, the FDA ruled that it was not a medical device — first successful pivot!
Risk #2: Technology
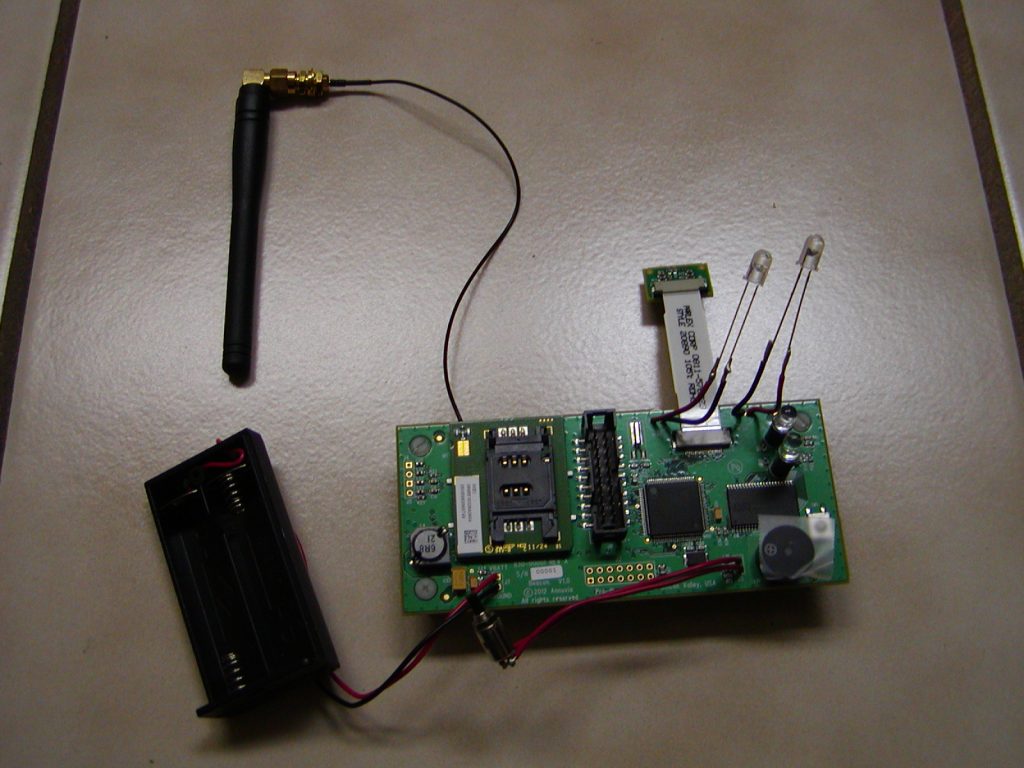
Sensors and Software Data Streaming
We initially tested the least expensive way to read the data from the AED: a light sensing diode that detects which icon is showing. It worked well in total darkness and direct sunlight. However, it obscured the display of the AED and was not stable when the cover moved. With testing, we determined that it was not technically feasible. This caused us to pivot and try a camera to take a picture of the display. Using this method, we arranged the camera and light source so the display screen was not obscured and was stabl. This worked well and was technically feasible — second successful pivot!
We also identified a software bottleneck for streaming the data. To mitigate this, the images were compressed down (to 10K bytes), thus making them economical to transmit, and we determined that the data was easy to extract.
Risk #3: Supply Chain
Display
The type of screen desired was custom (not off-the-shelf) due to size, resolution, and features required. To estimate the price, we had to compare similar screens with various resolutions and features. We determined that it was possible to get the screen desired using a semi-custom version that met our price and availability targets. This solved our third development risk.
Example: Feasibility Study – Technology Assessment
As a result of the feasibility assessment, we made a couple of successful pivots, that, if not identified and solved early, would have added over $100 K to the development cost and six months to the schedule, and resulted in a product that was not technically feasible and not meeting cost targets. If these issues were not discovered prior to volume production, the impact on the business could have been catastrophic. By performing a feasibility study first, we were able to understand key regulatory, technology, and supply chain options and associated risks BEFORE our client made a commitment to the full development project. The cost of development of the final project would have been hundreds of thousands of dollars. By spending a few thousand dollars up front, Annuvia was able to save $100K in unnecessary regulatory expense and bring functional technology and supply chains to bear on their product prior to costs and volumes escalating.
Following this feasibility assessment and prototype development, Annuvia was successfully acquired.
About Voler Systems
Voler Systems provides full-service R&D consulting from concept to moving new products into production with world class quality. Since 1979, clients have turned to us for reliable new products involving sensors and measurement electronics. Our multidisciplinary team delivers high-quality products on time and on budget. We have developed wearable devices, wireless testers, medical devices, automatic test equipment, instrumentation, data acquisition systems, and other specialized sensor-based electronics and prototype circuits.
About Product Realization Group
Product Realization Group is a consortium of Silicon Valley companies that can take your product from idea to scale. One source for prototype design and build, cost-reducing insights, and scalable manufacturing. Go to market faster, for less money than do-it-yourself. Get a smoother launch and lower costs by 20%. Our 500 clients include medical device, consumer electronic, and high-technology start ups, SMBs, and large companies such as GoPro, EMC, and Intuitive Surgical.


