
By UserWise
Imagine that an automated external defibrillator (AED) is on an airport wall and that a traveler goes into cardiac arrest. A passerby runs up to the AED, brings it to the person, and sets up the AED. They are about to resuscitate using an AED when they notice a blinking light with the label “Battery low.” The user thinks, “Oh, this isn’t going to work. I guess I shouldn’t use it,” and instead of using the machine, puts it aside. However, this is a misinterpretation of that indicator: they could have proceeded with using the AED to save that person’s life…but they did not.
This was a real-life situation resulting in the recall of this AED in 2009 due to the scenario described above. In this case a seemingly minor element of the design – the blinking “Battery low” light – could lead to the potential for death. The FDA-prescribed Human Factors process would have caught this issue before the AED ever made it onto the wall of that airport. This is the importance of human factors in the medical product development.
Human Factors Engineering is an important facet of product development, and is focused on minimizing or eliminating human error through the design of a product and its labeling. In the United States there are 40,000 deaths a year as a result of automobile accidents; that is, human error related to automobiles. By contrast, the U.S. loses 210,000 lives in hospitals due to preventable medical error. This number is a conservative estimate and pertains only to deaths within a hospital. However, the number increases as you consider medical error outside of the hospital as well, especially with more and more payers advocating for the home use of medical products. This is why human factors is important: it’s possible to design hospital processes and medical devices that minimize lives lost and maximize health to patients and users. Since 2016 when the Food & Drug Administration issued their final guidance, Applying Human Factors and Usability Engineering to Medical Devices, UserWise has seen an increase in the FDA’s enforcement of Human Factors in regulatory submissions.
By implementing human factors early in the medical device development process usability problems can be detected and addressed early, prior to making major investment in tooling and equipment and prior to negatively impacting regulatory submissions. Additionally, easier-to-use devices require less training and less customer support over the lifetime of the product. The human factors process can save substantial amounts of research and development time and money and long-term support costs, all while improving safety and ultimately customer satisfaction.
Early stage human factors research involves identifying design requirements by understanding the users and the use environments. User research provides direct input from intended users and explores environmental, social, and motivational aspects of the device design. Understanding the end users, including their knowledge base and education, as well as how they will behave at the point of use is critical to identifying potential use errors and the associated risks. By identifying usability problems early on through a use-related risk analysis that is informed by user research and usability testing, design efforts can be effectively prioritized to ensure that action is taken to prevent and mitigate the most serious use errors. User research also helps to identify an appropriate study environment and study model to best represent actual use during usability testing.
Formative usability testing is an iterative process that involves observation of intended users in a simulated-use environment with the product prototype to reveal potential use errors. During formative usability testing, subjective feedback on the design is also collected from study participants. In response to each study, rapid modifications can be made to the device design based on identified usability problems, including those arising from unexpected behavior of the intended users. It is a best practice to eliminate use errors by modifying the device design to prevent the use errors from happening. By conducting usability testing early and often in the product development process, design modifications can be easily made without having to invest the time and resources in making changes with a more developed product down the line.
In later stages of the development process, usability validation testing is used to demonstrate the safety and efficacy of the device with respect to usability. By optimizing the early design through formative usability testing, devices are less likely to experience unexpected validation results where features need to be modified and re-validated, resulting in higher costs and a likely delay in product introduction.
Figure 1: Cost of Product Development With and Without Human Factors
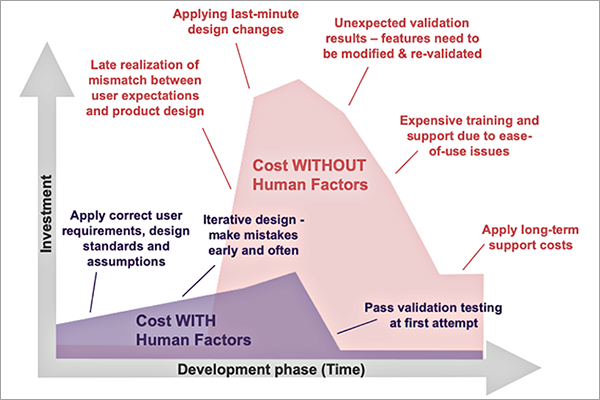
Incorporating human factors into the early stages of product development can yield great benefits and efficiency in bringing a product to market. Beyond market approval, a product designed with human factors in mind has additional benefits such as reducing the need for on-market training, reducing the risk of product recalls, and reducing long-term support and maintenance costs. Human factors is an essential part of the product development process that, when taken into consideration, can produce long-term benefits.

Learn more about Human Factors Engineering:
Contact us at 1-833-USERWISE to set up a free 1-hour Human Factors Consult or visit the UserWise Website for more information.


