While most of Silicon Valley moves at brakeneck speeds to ship product, those who produce medical devices are held back by the tethers of the regulatory process administered by the U.S. Food and Drug Administration (“FDA”). Similar requirements govern product approval for the CE mark (“European Conformity”) in European countries. However, while Europe regulates products for safety, the FDA also regulates products for efficacy, that is, effectiveness for intended use.
The FDA administers a very lengthy process which must be supported by the submission of scientific evidence through controlled studies. The FDA has explicit regulations and requirements about the studies, depending on the device classification. (See How to Study and Market Your Device).
Medical Product Development & Manufacturing – Expert Panel
The SV Hardware Startup to Scale Meetup had a panel on Medical Product Development & Manufacturing on April 4, 2017 at Evolve Manufacturing Technologies in Fremont, CA. Panelists include: Jordan Bajor, COO of Gynesonics, Inc., Tom Geraty, Senior Designer at Fusion Design™, and Noreen King, President and CEO of Evolve Manufacturing. We have included some key tips highlighted below:
Original Equipment Manufacturing (OEM) Advice
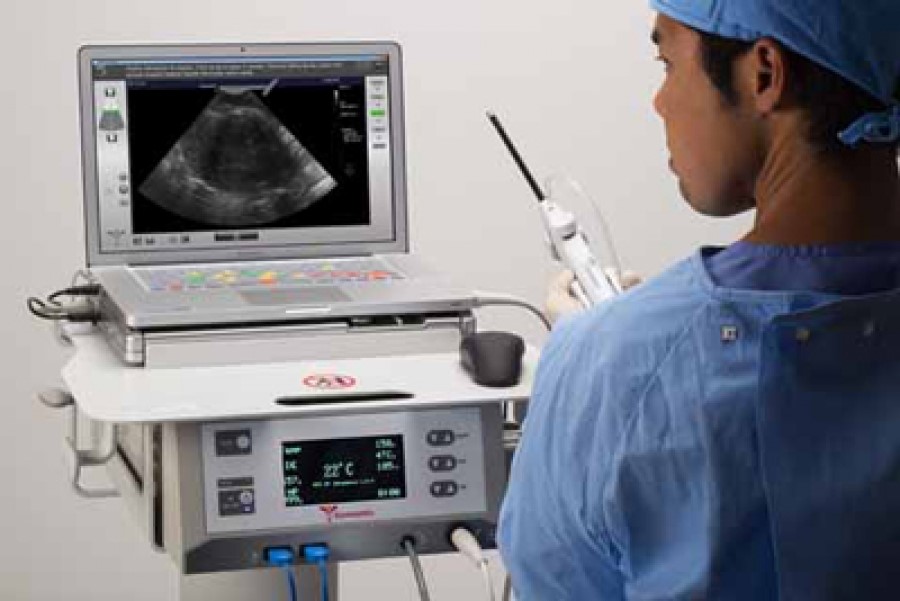
Jordan Bajor, a seasoned Medical Device veteran, joined the Gynesonics, Inc. team in 2005 as COO. Gynesonics is working on a product known as the the Sonata® System, which provides radiofrequency (RF) ablation of uterine fibroids using a transcervical approach without incisions. The Sonata System has a CE Mark and is approved for sale in the UK, Germany and the Netherlands. There is an enrolled study in the U.S. Gynesonics plans to file its 501(k) with the FDA with a goal to market the Sonata® System in the U.S. by the end of 2018. Regulations are not the only challenge in medical device development. When Gynesonics began work on the Sonata® System, women’s health was not a popular field for venture capital investment. According to Bajor, there has been a recent shift. Their funding consists of angel, venture as well as “strategic” investors.
To be successful in medical product development, you need more than a great idea. Bajor points to team, market, money and a well thought out business plan.
Drilling down on team, Bajor offers the following advice:
- Recruit a team with a proven track record.
- Bring in a seasoned CEO to run the company. You need a plan for the Founder, but not necessarily running the company.
- Have seasoned research and development (R&D) leadership to create product development processes.
- You don’t want to reinvent processes!
- You don’t want to create risks
- Get someone assigned to regulatory work early — from day 1.
Engineering Development Guidance
Fusion Design™ as a turnkey product and equipment development firm, has over 25 years of experience in industrial design, mechanical engineering and prototyping services.
In recent years, they have helped develop numerous medical devices, including:
VISX / Abbott Diagnostics Lasik; a laser eye surgery system
The IOne Touch Glucose Meter for Intuity

When Fusion Design™ worked with Abbott Diagnostics Lasik on VISX, it helped design a new enclosure for the device, a new user interface, and a new support for the patient. To better understand user needs, it built a full-scale, foam mock-up of the prototype to test the design features. Another type of prototype it does for clients are proof of concept (POC) models. In this instance it builds the early stage POC for the the customer to be able to sell an idea to investors.
Fusion Design™ gives the following development guidance:
- Accomplish the most difficult part of the design first
- Prototype early and often
- Have a regulatory expert as part of the team from the start
- Leverage your early prototypes to get customer and patient feedback
- Incorporate Design for Excellence (DFX) at each stage of your development process
Contract Manufacturing (CM) Tips
Evolve Manufacturing Technologies, Inc. is an established contract manufacturer in Fremont, led by Noreen King, President and CEO. They often work with medical device companies. Services they provide include design, manufacturing engineering, integration, testing, logistics, regulatory compliance and inventory management and stocking.
A recent manufacturing win for a medical device was a catheter inspection fixture for the customer’s product. Evolve supported build of a complex, temperature compensating water-bath fixture to adapt to their imaging system that worked perfectly upon delivery.
Evolve Manufacturing Technologies tips include:
- Engage your CM early in the development process
- Get your product documentation in order before transferring to suppliers
- Allow time in your development process to incorporate Design for Manufacturing (DFM) feedback
- Plan for production records keeping processes & costs to meet FDA regulations
- Treat your CM like a partner — they are an extension of your business!
Comments? Questions? Join the conversation on Facebook!


