When it comes to product development, implementing a robust process is critical to product success. There are different schools of thought – e.g., Waterfall, V-model, Agile – so how do you know which one will be the best for your medical device?
Traditional medical device product development process has often been done using the Waterfall methodology, which breaks everything into separate phases, each of which must be completed before you move onto the next phase. Product development is often also structured as shown in the graphic below.
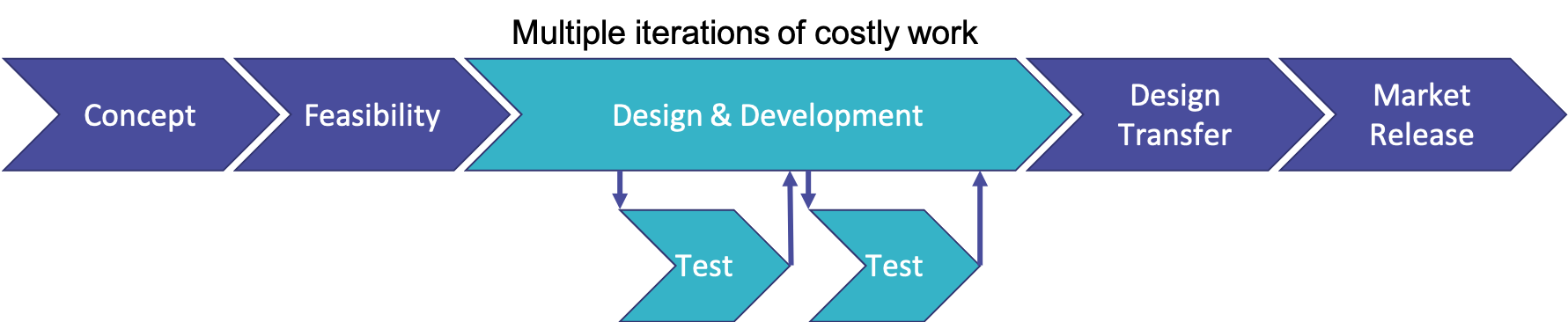
What is wrong with this picture? Most notably, it is that the design phase is often where the costliest work is done. Subsequent phases keep coming back to it as issues arise in those phases. This means that if there are problems with testing, during the product launch or in feedback received from customers, it all comes back to design, and around and around it goes again. This can lead to huge amounts of time and money wasted in repeating tasks and chasing dead ends.
So, how to solve this problem? One approach is to shift a lot of the work from being concentrated in the middle of the process to the beginning and to build in principles from Lean Product Development and Agile, versus Waterfall methodology.
From Waterfall to Agile
Lean Product Development is adapted from Toyota’s operating model “The Toyota Way.” Its key pillars are respect for people and continuous improvement. It places heavy emphasis on front-loading the product development process as well as short cycles and delivering value fast. The value (and entire method of thinking) tie back to the customer – addressing their problems and needs up front and at every phase. Lean is also similar to Agile which is a well-known development methodology for software. Like Lean, Agile also focuses on people, teamwork and continuous response and improvement throughout the process. The full Agile Manifesto can be found here.
Adopting Lean-Agile principles can be hugely beneficial to the medical product development process as it allows for multiple checkpoints and better collaboration. You can then stop and re-calibrate when something isn’t working – whether it is design, testing or the product not resonating with the customer. What is also critical, as mentioned earlier, is to move the heavy lifting to the front of the process. One way to do that is to include a “Phase Zero” during the concept phase. Phase Zero would incorporate the IDEO concept of “ideate, prototype, iterate” to quickly get to a technically de-risked design early in the development process and before the concept is finalized.
The process would look as follows:
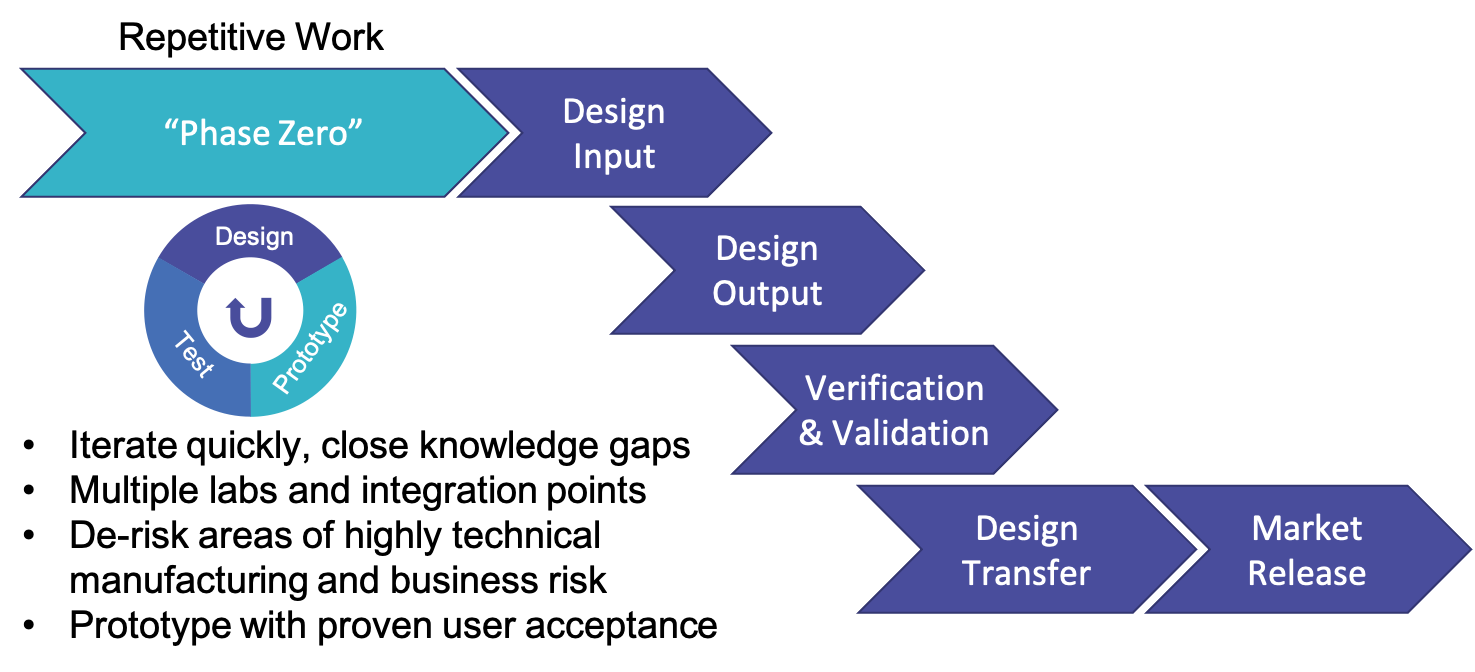
Phase Zero would allow for quick iteration and closing knowledge gaps and reduced risk.
I have personally seen this approach be very successful in multiple projects. From a MedTech perspective, specialists (for example, those who would be using the product – e.g. surgeons) can be brought into the process early for feedback and expertise which can directly feed into the product development. Prototypes can also be developed in this phase before the main design phase. The concentration of efforts in Phase Zero would also allow for lighter documentation releases. You can often develop a Minimum Viable Product (MVP) allowing you to submit for FDA clearance, while working in parallel on product commercialization. You can leverage early data for product justification and rationale versus having to wait post-testing to gather this information and then figure out how it can be used as input for product changes or launch.
Four Principles
There is no shortage of information on the different methodologies. If you are looking to bring a MedTech product to market, I would recommend you consider these four principles, above all:
- Understand your customer and their requirements – make sure the value of the proposed product to the customer is well-defined. It is helpful here to actually check in with this person – whether they are a doctor or someone else in the medical field.
- Ensure invention and the science is done in Phase Zero so you aren’t reinventing the wheel, so to speak, in design. This will help further mitigate risk.
- Have a process that allows you to iterate (e.g. use Agile/Lean). If it’s a big project, break it into smaller ones or defined milestones
- The people-first mentality extends beyond the customer – make sure you bring in all the key players as early in the process as possible (from engineering to marketing). This allows for more feedback, creative thinking, proactive tackling of future issues and more holistic product design.
By following these steps and building an Agile/Lean process into your approach, you can better create a product with strong customer demand, more efficiently meet regulatory requirements, and speed time-to-market.

Russ Singleton is an interim leader and advisor with a passion to maximize results for startups and turnarounds through critical inflection points. He does this by transforming leadership and culture, driving a customer-focused strategy, and partnering with technology teams for market-oriented innovation. As interim VP of R&D at Medrobotics, a surgical robotics startup, he was hired to jumpstart stalled efforts to secure CE Mark for a surgical robotics product. After successful approval, he joined full-time to lead transformation of the R&D organization. Prior to Medrobotics, Russ has extensive experience including CEO, COO, General Management and VP R&D in MedTech, Healthcare IT and Genomic Instrumentation companies. Russ holds a Ph.D. and M.S. in electrical engineering from the University of Illinois and a Bachelor of Engineering from the Pratt Institute.