INTRODUCTION: THE DIGITAL IMPERATIVE
In the fiercely competitive world of medical device manufacturing, the journey from prototype to commercialization is critical. Embracing digital transformation through Software as a Service (SaaS) based Product Lifecycle Management and electronic Quality Management Systems (PLM-eQMS) is not just an option but a necessity to get ahead. Implementing this early in the product lifecycle empowers companies with streamlined workflows, simplified regulatory compliance, and transparent operations across design, manufacturing, and supply chains.
Transformative Features of PLM-eQMS
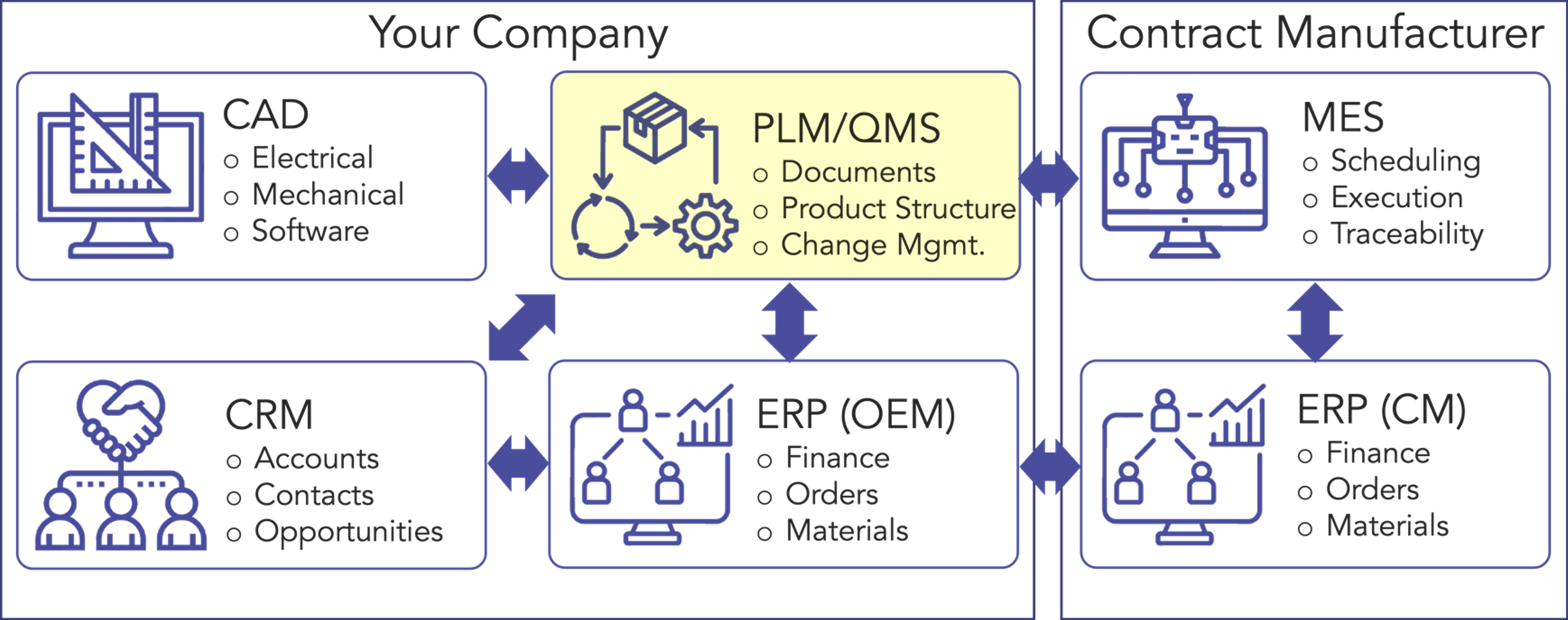
Cloud-based PLM-eQMS systems, deployable in just 2-3 months, offer tools that enable process optimization, control, and visualization vs. manual systems while simplifying regulatory compliance. By integrating with other systems, such as eCAD, mCAD, ERP, and CRM solutions, PLM-eQMS systems are key in building a company’s comprehensive information management system from product development through full-scale commercialization (Figure 1). The ability to electronically incorporate lean and agile methodologies to manage and break down complex systems into discrete, connected elements and tasks enhances collaboration of both local and remote teams, ensures risks are identified early and changes are implemented rapidly, thereby reducing rework and accelerating time-to-market.
The Business Perspective: Recognizing the Opportunity
In a traditional startup medical device environment, upstream process inefficiencies magnify downstream issues, leading to significant delays and missed milestones. These avoidable scenarios may be prevented when starting out using digital systems. The key is to ‘start with the end in mind’ from a systems perspective. Working backward from company strategic, development, regulatory, and commercial goals is a valuable first step. Also, understanding IT infrastructure plans and budget constraints is vital to choosing the right PLM-eQMS solution and setting implementation priorities.
Common business drivers for implementing business systems include the following:
- Enabling faster product development and introduction.
- Supporting rapid development iterations and greater innovation.
- Improving data accuracy and integrity (reducing manual entry).
- Searching for and accessing data more effectively and faster.
- Automating manual processes and implementing workflows.
- Integrating and automating the flow of data between systems.
- Complying with ISO, (EU) MDR, and FDA regulations (using electronic signoff).
- Supporting more effective project management.
- Enabling real-time data visualization of key performance indicators.
- Maximizing your budget.
Case Study: Streamlining Operations for a Startup
Consider a startup developing its first product. Managing document revisions is challenging, and the company struggles with missing or disconnected requirements discovered during verification testing. As a result, the startup has many design rework cycles. The organization recognized it needed to stop using an ad-hoc product development approach. So, they decided to implement an electronic PLM-eQMS system first to achieve ISO 9001 and then ISO ISO 13485 certifications.
The Solution: integrating PLM-eQMS functionality
The solution to solving the complex system requirements management, verification and validation, and risk management were:
- Instituting document control
- Adopting a Minimum Viable Product (MVP) requirements approach
- Applying agile methods to product development
Starting with these foundational quality processes illustrates the strategic value of integrating PLM-eQMS functionality early in the startup business lifecycle.
Implementing the Solution: A Structured Approach
Selecting the right PLM-eQMS involves stakeholder interviews, gap analysis, and vendor evaluations to meet a company’s unique needs. The product development goal was a high priority and needed system features enabling Agile development, Scrum practices, block diagram system architectures, and project management to uniquely account for and relate to and manage every part, requirement, risk, test, and associated document.
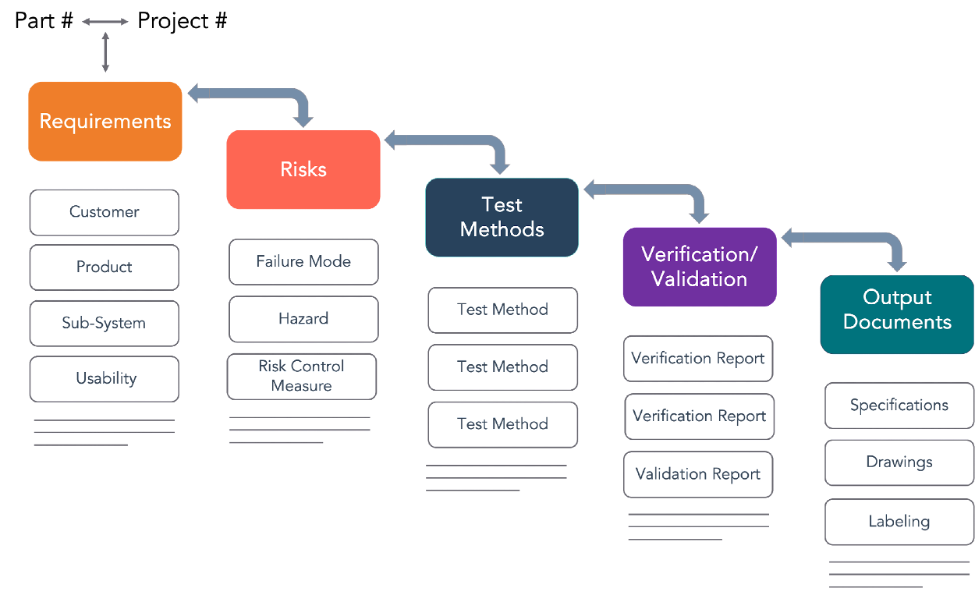
Figure 2 shows typical design control trace matrix element relationships configurable as a report to manage requirement complexity with minimum maintenance overhead. Although product development was the top business priority, the need to have document structure and change control was critical; therefore, documentation–including drawings and Bills of Materials (BOMs) and change control–was selected as the first PLM-eQMS implementation areas followed by product development.
This two-step approach provided the foundational systems to build all other areas. Based on the implementation plan priorities, future processes such as complaints, CAPA, training, supplier management, audit, issue management, quality control, and non-conforming materials management could be implemented within a few weeks each.
Conclusion: Realizing Immediate and Long-term Benefits
Implementing a cloud-based PLM-eQMS system with agile development methods yields immediate benefits. The result is faster development cycles, document and engineering change cycles, quicker resolution of manufacturing defects and customer complaints, and efficient collaboration of dispersed teams. These improvements lead to streamlined product development and operations and pave the way for growth, scalability, and simplified regulatory compliance.
Tips for Successful Business Systems Deployment
- Develop an end-to-end systems strategy, considering outsourced partners and data flows between systems.
- Secure management support before investing time and resources.
- Utilize Software as a Service (SaaS) platforms for scalability and ease of management.
- Allocate time and budget for process definition, training, and automation of data exchange to improve efficiency.
- Appoint a dedicated project manager and provide ongoing support resources.
- Establish clear Statements of Work (SOWs) and create service-level agreements with software vendors.
- Ensure ample end-user training and reserve a budget for vendor support and systems update management.
For more information on leveraging agile PLM-eQMS systems to bring your products to market efficiently and at scale, contact the Product Realization Group.
If you enjoyed this article, you may also enjoy viewing our Ask The Expert series
- Why is the leading cause of new product delays compliance failure?
- Why is failing to adhere to regulatory compliance one of the biggest challenges when bringing a medical device to market?
Or check out our website to learn more about PRG’s Med-NPI IMPACT and our client’s success: Med-NPI IMPACT Enables FDA Compliance & Full-scale Manufacturing for Debut Product.
About the author
Jeff Cody, President, Cascade Quality Services, LLC.
With over two decades of experience, Jeff consistently deliveres sustainable and scalable compliance solutions globally in the MedTech industry. As a Quality Leader, he led acquisition integrations, scaled up manufacturing and supply chain capabilities, and contributed to launching many new products in heart failure, diagnostic and life science consumables, and capital equipment.
His expertise includes improving and installing new ISO13485 Quality Management Systems and integrating Lean Methods, Agile Thinking, Dfx, and Data Analytics in large companies and start-up organizations to meet evolving regulatory requirements.
Jeff recently founded a consulting company, Cascade Quality Services LLC., to deliver a competitive edge to companies using electronic PLM-eQMS systems to reduce design cycle times and to realize the benefits of a connected and collaborative Quality System. Jeff has a Master’s Degree in Electrical Engineering from Arizona State University.